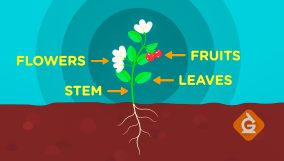
Energy Transfer Definition
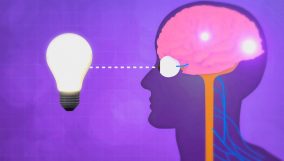
Energy transfer is the movement of energy between objects or regions. For example, heating a metal rod at one end causes energy to move to the cooler end by conduction.
View Lesson on Heat: Transfer of Thermal Energy
Become a member to get full access to our entire library of learning videos, reading material, quiz games, simple DIY activities & more.
Become a member to get full access to our entire library of learning videos, quiz games, & more.
Plans & Pricingto watch this full video.

Access All Videos
and Lessons, No Limits.
Access All Videos

No credit card required,
takes 7 sec to signup.
No card required

Ready-to-go lessons
that save you time.
Ready-to-go lessons
If you are on a school computer or network, ask your tech person to whitelist these URLs:
*.wistia.com, fast.wistia.com, fast.wistia.net, embedwistia-a.akamaihd.net
Sometimes a simple refresh solves this issue. If you need further help, contact us.
Heat: Transfer of Thermal Energy
Fun Facts
- If an object feels cold to our touch, it's because thermal energy was transferred from our finger to the object.
- The amount of a substance is one factor that affects the rate of thermal energy transfer.
- Using different materials can slow or speed up the rate of thermal energy transfer.
Why Do We Need To Know About Energy Transfer
Learning about how energy moves helps us understand how things around us work and why it’s important in many jobs. For example, our clothes keep us warm by stopping heat from escaping, and solar cookers use sunlight to cook food. This idea of energy transfer is used in many ways.
It’s also used to keep people warm in emergencies with special blankets, make hot air balloons fly by heating air, and stop computers from getting too hot. Knowing about energy transfer can make us smarter about the world and lead to jobs in things like green energy, air conditioning and heating, and making food.
Frequently Asked Questions
Check out the Full Lesson on Heat: Transfer of Thermal Energy
In this lesson, we learn that:
- Heat is the transfer of thermal energy from one object to another.
- Heating can occur by conduction, convection and radiation.
- Some materials can store more thermal energy than others.
Related Topics
- Absorbency Definition
- Balanced Force Definition
- Biodiversity Definition
- Camouflage Definition
- Carnivore Definition
- Chloroplasts Definition
- Classify Definition
- Coastal Erosion Definition
- Computer Programming Definition
- Conduction Definition
- Convection Definition
- Corona Definition
- Decomposer Definition
- Definition Of Experiment
- Definition Of Nutrients
- Digestive System Definition
- Distillation Definition
- Energy Transfer Definition
- Engineer Definition
- Environment Definition
- Freezing Definition
- Frequency Definition
- Greenhouse Effect Definition
- Heat Definition
- Humidity Definition
- Magnetism Definition
- Meteorologist Definition
- Moon Definition
- Muscular System Definition
- Mutualism Definition
- Natural Selection Definition
- Offspring Definition
- Particle Model Of Matter Definition
- Physical Change Definition
- Pollen Definition
- Predation Definition
- Properties Of Matter Definition
- Rain Definition
- Sedimentary Rock Definition
- Seed Dispersal Definition
- Seismologist Definition
- Snow Definition
- Solar Eclipse Definition
- Temperature Definition
- Thermal Energy Definition
- Trait Definition
- Tsunami Definition
- Wind Erosion Definition
Start a Free Trial Today. Get a $5 Amazon Gift Card!
Teachers! Start a free trial & we'll send your gift card within 1 day. Only cards left. Try it now.
Select Grade
Select Subject
This email is associated with a Science Kit subscription. Kit subscriptions are managed on this separate page: Manage Subscription

-
Download InvoiceScience & Math$/yr
-
Download InvoiceScience Only$/yr

access all lessons
• No credit card required •
"My students loved the videos. I started the video subscription in May and used them as a review before the state test, which I know contributed to 100% of my class passing the state test."
Rhonda Fox 4th Grade Teacher, Ocala, Florida
• No credit card required •
"My students loved the videos. I started the video subscription in May and used them as a review before the state test, which I know contributed to 100% of my class passing the state test."
Rhonda Fox 4th Grade Teacher, Ocala, Florida
• No credit card required •
Already a member? Sign In
* no credit card required *

* no credit card required *
* no credit card required *


no credit card required
Skip, I will use a 3 day free trial
Enjoy your free 30 days trial
-
Unlimited access to our full library
of videos & lessons for grades K-5. -
You won’t be billed unless you keep your
account open past your 14-day free trial. -
You can cancel anytime in 1 click on the
manage account page or by emailing us.
-
Unlimited access to our full library of videos & lessons for grades K-5.
-
You won't be billed unless you keep your account open past 14 days.
-
You can cancel anytime in 1-click on the manage account page.
Cancel anytime in 1-click on the manage account page before the trial ends and you won't be charged.
Otherwise you will pay just $10 CAD/month for the service as long as your account is open.
Cancel anytime on the manage account page in 1-click and you won't be charged.
Otherwise you will pay $10 CAD/month for the service as long as your account is open.
We just sent you a confirmation email. Enjoy!
DoneWe use cookies to make your experience with this site better. By using this site you agree to our use of cookies. Click "Decline" to delete and block any non-essential cookies for this site on this specific property, device, and browser. Please read our privacy policy for more information on the cookies we use.Learn More
We use cookies to improve your experience. By using this site, you agree to our use of cookies. Click "Decline" to block non-essential cookies. See our privacy policy for details.Learn More