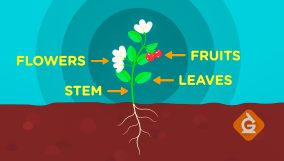
Physical Change Definition
A physical change involves no new substance. For example, boiling water changes state but remains water.
View Lesson on Chemical vs. Physical Changes
Become a member to get full access to our entire library of learning videos, reading material, quiz games, simple DIY activities & more.
Become a member to get full access to our entire library of learning videos, quiz games, & more.
Plans & Pricingto watch this full video.

Access All Videos
and Lessons, No Limits.
Access All Videos

No credit card required,
takes 7 sec to signup.
No card required

Ready-to-go lessons
that save you time.
Ready-to-go lessons
If you are on a school computer or network, ask your tech person to whitelist these URLs:
*.wistia.com, fast.wistia.com, fast.wistia.net, embedwistia-a.akamaihd.net
Sometimes a simple refresh solves this issue. If you need further help, contact us.
Chemical vs. Physical Changes
Fun Facts
- Combining milk and cereal for breakfast is a physical change.
- Mixing Mentos with soda is a physical change because no new substance is made.
- It is usually easy to reverse a physical change, like dissolving sugar in water then evaporating the water to leave the sugar crystals behind.
Why Do We Need To Know About Physical Change
Learning about physical changes helps us know why different materials act the way they do and why changing them is important for lots of jobs. For example, knowing how a metal like Gallium can melt and solidify without turning into a different substance helps in making things from everyday items to high-tech gadgets.
This knowledge isn’t just for scientists but for everyone. When you whip egg whites to make them fluffy for cooking, that’s a physical change. Also, in things like cleaning water or designing eco-friendly projects, using physical changes is key. Understanding physical changes is useful for many reasons.
Frequently Asked Questions
Check out the Full Lesson on Chemical vs. Physical Changes
In this lesson, we learn that:
- In a chemical change we form an entirely new substance.
- In a physical change, we don't form a new substance.
- Physical changes also occur when matter changes states.
Related Topics
- Absorbency Definition
- Carbon Dioxide Definition
- Cast Fossils Definition
- Chemical Change Definition
- Chemical Reaction Definition
- Circuit Definition
- Conservation Definition
- Corona Definition
- Definition Of Experiment
- Definition Of Non-living Things
- Definition Of Science
- Dichotomous Key Definition
- Distillation Definition
- Ecosphere Definition
- Evaporation Definition
- Exoskeleton Definition
- Frequency Definition
- Gas Definition
- Geosphere Definition
- Greenhouse Gases Definition
- Hydrosphere Definition
- Internal Structures Definition
- Kuiper Belt Definition
- Latitude Definition
- Light Reflection Definition
- Light Source Definition
- Liquid Definition
- Marsupial Definition
- Melting Definition
- Nervous System Definition
- Physical Change Definition
- Predation Definition
- Predator Definition
- Prey Definition
- Renewable Resource Definition
- Respiratory System Definition
- River Definition
- Signal Definition
- Simple Machines Definition
- Solid Definition
- Star Definition
- Sun Definition
- Symbiosis Definition
- Unbalanced Force Definition
- Virus Definition
- Volcano Definition
- Wave Definition
- Wind Erosion Definition
Start a Free Trial Today. Get a $5 Amazon Gift Card!
Teachers! Start a free trial & we'll send your gift card within 1 day. Only cards left. Try it now.
Select Grade
Select Subject
This email is associated with a Science Kit subscription. Kit subscriptions are managed on this separate page: Manage Subscription

-
Download InvoiceScience & Math$/yr
-
Download InvoiceScience Only$/yr

access all lessons
• No credit card required •
"My students loved the videos. I started the video subscription in May and used them as a review before the state test, which I know contributed to 100% of my class passing the state test."
Rhonda Fox 4th Grade Teacher, Ocala, Florida
• No credit card required •
"My students loved the videos. I started the video subscription in May and used them as a review before the state test, which I know contributed to 100% of my class passing the state test."
Rhonda Fox 4th Grade Teacher, Ocala, Florida
• No credit card required •
Already a member? Sign In
* no credit card required *

* no credit card required *
* no credit card required *


no credit card required
Skip, I will use a 3 day free trial
Enjoy your free 30 days trial
-
Unlimited access to our full library
of videos & lessons for grades K-5. -
You won’t be billed unless you keep your
account open past your 14-day free trial. -
You can cancel anytime in 1 click on the
manage account page or by emailing us.
-
Unlimited access to our full library of videos & lessons for grades K-5.
-
You won't be billed unless you keep your account open past 14 days.
-
You can cancel anytime in 1-click on the manage account page.
Cancel anytime in 1-click on the manage account page before the trial ends and you won't be charged.
Otherwise you will pay just $10 CAD/month for the service as long as your account is open.
Cancel anytime on the manage account page in 1-click and you won't be charged.
Otherwise you will pay $10 CAD/month for the service as long as your account is open.
We just sent you a confirmation email. Enjoy!
DoneWe use cookies to make your experience with this site better. By using this site you agree to our use of cookies. Click "Decline" to delete and block any non-essential cookies for this site on this specific property, device, and browser. Please read our privacy policy for more information on the cookies we use.Learn More
We use cookies to improve your experience. By using this site, you agree to our use of cookies. Click "Decline" to block non-essential cookies. See our privacy policy for details.Learn More