Proton Definition
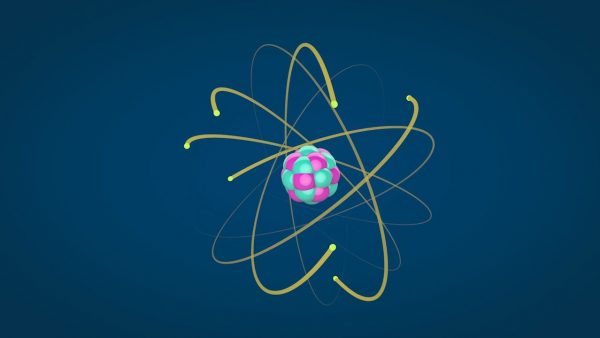
A proton is a positively charged particle in an atom's nucleus. For example, a hydrogen atom has one proton in its nucleus.
View Lesson on Atoms & Molecules
Become a member to get full access to our entire library of learning videos, reading material, quiz games, simple DIY activities & more.
Become a member to get full access to our entire library of learning videos, quiz games, & more.
Plans & Pricingto watch this full video.

Access All Videos
and Lessons, No Limits.
Access All Videos

No credit card required,
takes 7 sec to signup.
No card required

Ready-to-go lessons
that save you time.
Ready-to-go lessons
If you are on a school computer or network, ask your tech person to whitelist these URLs:
*.wistia.com, fast.wistia.com, fast.wistia.net, embedwistia-a.akamaihd.net
Sometimes a simple refresh solves this issue. If you need further help, contact us.
Atoms & Molecules
Fun Facts
- Protons are positively charged and located in the center of an atom.
- Carbon has 6 protons, 6 electrons, and 6 neutrons.
- The atomic number on the Periodic Table indicates an element's number of protons.
Why Do We Need To Know About Proton
Learning about protons helps us understand matter. This is important for scientists who make new medicines or figure out how to fight diseases. For example, when they understand what is inside an atom, they can make molecules that our bodies can use to heal.
By studying how our bodies work at the atomic level, scientists can understand how things like proteins do their job. Proteins make your heart beat, give your hair its color and more. This knowledge is also behind making insulin proteins for people with diabetes. So, understanding the definition of a proton is really important for keeping us healthy and understanding how life works.
Frequently Asked Questions
Check out the Full Lesson on Atoms & Molecules
In this lesson, we learn that:
- Everything around us is made of atoms and molecules.
- An atom is the basic unit of an element. It consists of protons, neutrons and electrons.
- Atoms can combine to form molecules as simple as water or as complex as DNA.
Related Topics
- Algae Definition
- Astronomy Definition
- Atom Definition
- Balanced Force Definition
- Biochemistry Definition
- Biosphere Definition
- Body Fossils Definition
- Circuit Definition
- Comparative Anatomy Definition
- Competition Definition
- Conservation Biologist Definition
- Corona Definition
- Definition Of Evidence
- Ecosphere Definition
- Electric Charge Definition
- Electric Field Definition
- Electron Definition
- Engineer Definition
- Extinct Definition
- Food Web Definition
- Gas Definition
- Genetic Factors Definition
- Geologic Time Scale Definition
- Geosphere Definition
- Insulator Definition
- Internal Structures Definition
- Light Reflection Definition
- Magma Definition
- Matter Definition
- Meteorologist Definition
- Molecule Definition
- Nonrenewable Resource Definition
- Paleontologist Definition
- Plant Definition
- Pollution Definition
- Properties Of Matter Definition
- Property Definition
- Proton Definition
- Recycle Definition
- Renewable Resource Definition
- Salt Water Definition
- Simple Machines Definition
- Solar Eclipse Definition
- Sun Definition
- Sunlight Definition
- Translucent Definition
- Water Cycle Definition
- Wedge Definition
Start a Free Trial Today. Get a $5 Amazon Gift Card!
Teachers! Start a free trial & we'll send your gift card within 1 day. Only cards left. Try it now.
Select Grade
Select Subject
This email is associated with a Science Kit subscription. Kit subscriptions are managed on this separate page: Manage Subscription

-
Download InvoiceScience & Math$/yr
-
Download InvoiceScience Only$/yr

access all lessons
• No credit card required •
"My students loved the videos. I started the video subscription in May and used them as a review before the state test, which I know contributed to 100% of my class passing the state test."
Rhonda Fox 4th Grade Teacher, Ocala, Florida
• No credit card required •
"My students loved the videos. I started the video subscription in May and used them as a review before the state test, which I know contributed to 100% of my class passing the state test."
Rhonda Fox 4th Grade Teacher, Ocala, Florida
• No credit card required •
Already a member? Sign In
* no credit card required *

* no credit card required *
* no credit card required *


no credit card required
Skip, I will use a 3 day free trial
Enjoy your free 30 days trial
-
Unlimited access to our full library
of videos & lessons for grades K-5. -
You won’t be billed unless you keep your
account open past your 14-day free trial. -
You can cancel anytime in 1 click on the
manage account page or by emailing us.
-
Unlimited access to our full library of videos & lessons for grades K-5.
-
You won't be billed unless you keep your account open past 14 days.
-
You can cancel anytime in 1-click on the manage account page.
Cancel anytime in 1-click on the manage account page before the trial ends and you won't be charged.
Otherwise you will pay just $10 CAD/month for the service as long as your account is open.
Cancel anytime on the manage account page in 1-click and you won't be charged.
Otherwise you will pay $10 CAD/month for the service as long as your account is open.
We just sent you a confirmation email. Enjoy!
DoneWe use cookies to make your experience with this site better. By using this site you agree to our use of cookies. Click "Decline" to delete and block any non-essential cookies for this site on this specific property, device, and browser. Please read our privacy policy for more information on the cookies we use.Learn More
We use cookies to improve your experience. By using this site, you agree to our use of cookies. Click "Decline" to block non-essential cookies. See our privacy policy for details.Learn More