When something burns up, the matter does not vanish. The materials simply turn into gases you cannot see.
Matter never vanishes.

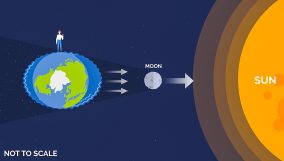
Matter is anything that has weight and takes up space. Anything you can see and touch is matter. Remember, matter has three main forms: solid, liquid, and gas.
Matter can change from one form to another. For example, water can be boiled, which turns it into a gas. It might seem like the boiling water vanished, but it just changed into a form we cannot see, called water vapor.


























































































































 GENERATION GENIUS
GENERATION GENIUS




