Sulfur hexafluoride is denser than air. When a foil boat was placed in a tank of sulfur hexafluoride, the boat floated. That’s because the air in the boat is less dense than the sulfur hexafluoride gas in the fish tank.
Matter is anything that has weight and takes up space. Everything you can see and touch is made up of matter. Matter exists in three main forms: solids, liquids, and gases. It also has properties that we can describe through density, solubility, conductivity, magnetism, etc.
To better understand the properties of matter…
LET’S BREAK IT DOWN!
When scientists use the word “matter” they are talking about solids, liquids, and gases.

Matter can be found on Earth in three main forms: solids, liquids, and gases. Solids are materials that have a defined shape and volume that stays the same. Rocks are a good example of a solid - they have a rigid shape that isn’t easily changed.
Liquids are a type of matter that changes shape depending on the shape of its container. For example, when you pour milk into a cup, it takes up the cup’s inner shape.
Matter that spreads out to take up all the space available in the container is called a gas. Air is a gas. So is helium, which is put inside birthday party balloons.
Matter can be identified through its properties.

One clue to helps us identify matter is magnetism. Magnetism is the ability of a material to be attracted by a magnet. Only certain materials are attracted to magnets, like iron, nickel, and cobalt.
Another property that can help us identify matter is solubility. Solubility describes how well a substance can be dissolved. Some substances, like salt, are easily dissolved by water but not easily dissolved by other liquids, like acetone. Acetone is a chemical found in nail polish remover. Acetone does a great job dissolving nail polish, but it cannot dissolve salt.
Density is an important property of matter.

An object’s density depends on how closely the tiny particles are packed together. Objects with a high density have particles that are more tightly packed than objects with a low density.
To better understand density you can think about the difference between a golf ball and a ping-pong ball. Even though they are about the same size, golf balls are heavier because they have a higher density.
How something floats or sinks is also related to its density. In the video, one balloon was filled with helium and the other was filled with sulfur hexafluoride. The helium balloon went up because its density is less than air. The balloon with sulfur hexafluoride sank because its density is greater than air.
Knowing the properties of matter can help you pick the right materials for the job.

If you are going on a canoe trip and want to take along some cold sodas, taking a Styrofoam cooler would be a good choice of materials. Styrofoam is not dissolved by water and is a good insulator. However, if you wanted to store some acetone for a science project, a Styrofoam container would not be a good choice. Acetone easily dissolves Styrofoam, meaning it would melt through.
If you are making a rocket engine, it might seem like a good idea to make it out of aluminum because it is a light metal, however aluminum would also melt from the rocket's heat. In this case, you might want to choose ceramic (same thing pottery is made of) because it has better properties (withstands heat).
PROPERTIES OF MATTER EXAMPLES


Handles on pots do not conduct heat. Making handles out of plastic prevents the handle from becoming too hot to touch.

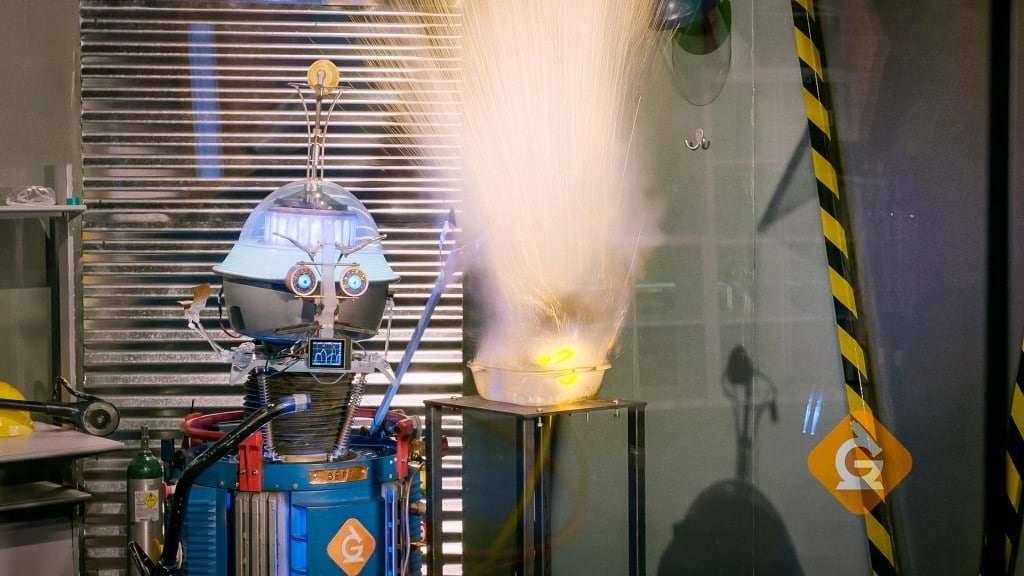
Sodium metal creates an exciting reaction with water. In the video we identified which metal was sodium by using a magnet. Sodium also has some interesting chemical properties — it bursts into flames when put in water!
PROPERTIES & FORMS OF MATTER VOCABULARY
PROPERTIES & FORMS OF MATTER DISCUSSION QUESTIONS
Which property did Zoe use to figure out which metal was sodium and which was iron?
Which is more dense: sulfur hexafluoride or helium? How do you know (what evidence did you see)?
What properties of propane make it a good choice for grilling?
What properties make metal a bad choice of material for the handle of a pot?
What properties make stainless steel a better choice for a knife and fork than Swiss cheese?
What are some properties that can be used to describe solid, liquid and gases?
What are the properties and common uses of water in each of its states?
Liquid water is non-magnetic and non-conductive. It is used for drinking, washing dishes and watering plants.
Water in the gas phase is also non-magnetic and non-conductive. It has a much lower density than liquid water. It is used for powering steam engines and some power plants.
Water in the solid phase (ice) is also non-magnetic and non-conductive. Surprisingly, it has a slightly lower density than liquid water. This is a unique property of ice and it is the reason that ice floats on water. If it didn't, all the fish in a frozen lake would get crushed! Ice is used to build igloos, keep food cold and to reduce swelling if you have an injury.
Why is knowing properties of matter important for engineers?
Skip, I will use a 3 day free trial
Enjoy your free 30 days trial





