
Elements are pure substances made of only one type of atom. An atom is made of protons, neutrons, and electrons. For example, a carbon atom has 6 protons, 6 neutrons, and 6 electrons. This gives carbon unique properties that can be used to identify it. There are 118 naturally occurring elements, each one has a different number of protons.
To better understand the properties of elements…
LET’S BREAK IT DOWN!
Elements are made of the same types of atoms.

Elements are naturally occurring substances that exist in all three states—solid, liquid, and gas. Elements are made of the same types of atoms. For example, oxygen exists naturally as a diatomic element, meaning that it has two oxygen atoms. Each atom is made of protons, neutrons, and electrons. Protons and neutrons are positioned in the center of the atom called the nucleus, and electrons are found in the outer portions of the atom called energy levels, or electron orbitals.
All elements have properties.

All elements have properties. Those properties include, but are not limited to, conductivity, magnetism, melting point, boiling point, color, state of matter, and others. Elements with similar properties are grouped together in different areas of the periodic table of elements. Observing an element’s properties allows scientists to categorize it and also determine its reactivity with other elements. Where the element is positioned on the periodic table will determine how it combines, or doesn’t, with other elements.
Elements are arranged in a pattern on the periodic table.
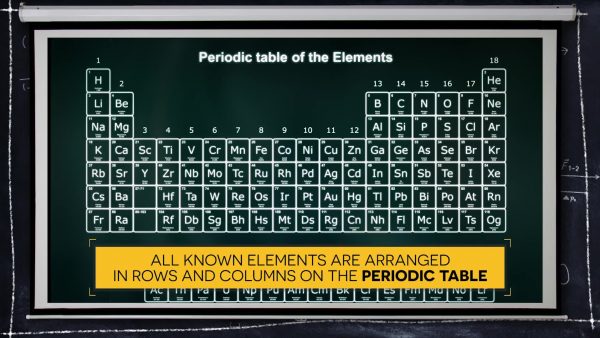
The pattern in which elements are arranged on the periodic table helps scientists categorize them based on properties and reactivity. Each period (row) of the periodic table is associated with the number of energy levels that orbit a nucleus. Row 1 has one energy level; Row 2 has two energy levels; Row 3 has three energy levels, and so on, up to 7. The number of electrons orbiting the nucleus of an atom will determine how that element interacts with other elements.
Different groups of elements have different properties.

The properties of elements, including but not limited to density and reactivity, can be predicted based on their arrangement in the periodic table. For example, as you move across a period, density usually increases. Because of this, an equal sized cube of titanium, iron, and copper may all have the same volume but will have different densities. Other properties of elements can be predicted from the periodic table as well including state of matter, reactivity, and conductivity.
Elements are in our everyday lives.

The element lithium is found in lithium batteries that power your phones, computers, and nearly all rechargeable devices. Fluorine atoms can be found in drinking water and toothpaste. It fights cavities and tooth decay. Calcium is needed to form bones and the shells of living things. Bismuth is found in medicine to treat diarrhea and helps people to feel better from an upset stomach. Iodine is used as a skin disinfectant, especially, when someone has surgery. Chromium is used to make shiny rims on new cars. Copper is used inside electrical wire to conduct electricity.
PROPERTIES OF ELEMENTS VOCABULARY
PROPERTIES OF ELEMENTS DISCUSSION QUESTIONS
What do the rows of the periodic table indicate?
Give two examples of where you would find elements in our everyday lives.
How can you use the periodic table to help predict reactivity?
What are noble gases?
Skip, I will use a 3 day free trial
Enjoy your free 30 days trial





